摘要:本文解释了如何用Gaussian计算化合物(AH)在水溶液中的酸度(pKa)。
一. pKa计算的热力学循环
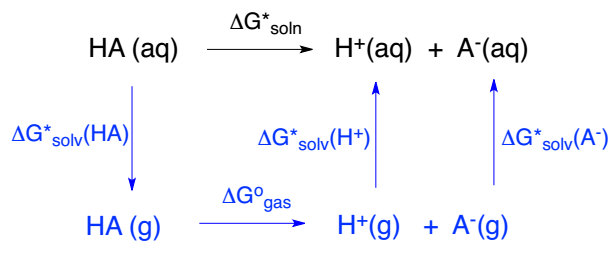
Figure 1. pKa计算的热力学循环,改编自Matthew(2001)的cycle 1.
根据Figure 1所述循环,可得pKa的计算公式如下:
|
ΔGsoln = ΔGgas + ΔGsolv(H+) + ΔGsolv(A–) – ΔGsolv(HA) |
(1) |
|
pKa = ΔG*soln/RTln(10) |
(2) |
|
ΔG*soln = ΔGsoln + Δn x 1.89 |
(3) |
Matthew(2001)用Cycle 1计算pKa时,Ggas(H+)与ΔGsolv(H+)采用了实验值,其它的气体自由能用CBS或G-n计算,溶剂化自由能用HF/6-31g(d)组合CPCM计算,则有下面公式(4)(原文方程8)直接计算pKa:
|
pKa = [Ggas(A–) – Ggas(HA) + ΔGsolv(A–) – ΔGsolv(HA) – 269.0]/1.3644 |
(4) |
其中气相自由能(Ggas)可以采用CBS或G-n计算,溶剂化自由能(ΔGsolv)可以参考我们之前的教程。
- 气相自由能(Ggas)用CBS或G-n精确计算,参考Gaussian使用手册
- 溶剂化自由能(ΔGsolv)计算请参考我们的教程
二. 计算过程
以甲酸的酸度计算为例:
HCOOH -> HCOO– + H+
根据Matthew(2001)的Cycle 1方法,分别计算HCOOH、HCOO–、H+的气相自由能与溶剂化自由能,即可用来预测pKa。在本文中,气相自由能用CBS-QB3计算、溶剂化自由能用HF/6-311+g(2d,p)组合SMD溶剂化模型计算。计算结果见下表:
| Items | Energy | Unit |
|---|---|---|
| Ggas(HCOOH) | -189.551515 | Hatree |
| Ggas(HCOO–) | -189.00582 | Hatree |
| Ggas(H+) | -6.28 | kcal/mol |
| ΔGsolv(HCOOH) | -10.03 | kcal/mol |
| ΔGsolv(HCOO–) | -77.69 | kcal/mol |
| ΔGsolv(H+) | -264.61 | kcal/mol |
注:其中Ggas(H+)与ΔGsolv(H+)采用的是实验值。
因此有:
ΔGgas=Ggas(HCOO–)+Ggas(H+)-Ggas(HCOOH)=336.15kcal/mol
ΔΔGsolv=ΔGsolv(HCOO–)+ΔGsolv(H+)-ΔGsolv(HCOOH)=-332.27kcal/mol
ΔG*soln = ΔGgas + ΔΔGsolv + 1 x 1.89 = 5.76kcal/mol
pKa=ΔG*soln/RTln(10) = (5.76 x 4184)/(8.314 x 298.15 x ln(10) = 4.22
HCOOH在室温的pKa为3.75,实验值与计算值的差值为0.5。
最简单的方法是用Matthew(2001)的方程8(本文的公式4)直接计算:
pKa=[Ggas(A–)-Ggas(HA)+ΔGsolv(A–)-ΔGsolv(HA)-269.0]/1.3644
=(-189.00582*627.509+189.551515*627.509-77.69+10.03-269)/1.3644
=4.23
两者计算结果基本一样。
四. 算例(全部由Gaussian 16, Revision A.03完成计算)
甲酸气相自由能计算
1 2 3 4 5 6 7 8 9 10 11 12 13 | %chk=formic_CBS.chk #p CBS-QB3 Acid CBS 0 1 C -0.13383400 0.39594000 0.00002200 O -1.13283000 -0.26239400 -0.00000900 O 1.11497600 -0.08928500 -0.00000700 H -0.10360900 1.49434600 0.00003500 H 1.04945100 -1.05655300 -0.00003800 !任何空白行不可省略 |
结果:
1 2 3 4 5 6 7 8 9 10 11 12 13 14 | Complete Basis Set (CBS) Extrapolation: M. R. Nyden and G. A. Petersson, JCP 75, 1843 (1981) G. A. Petersson and M. A. Al-Laham, JCP 94, 6081 (1991) G. A. Petersson, T. Tensfeldt, and J. A. Montgomery, JCP 94, 6091 (1991) J. A. Montgomery, J. W. Ochterski, and G. A. Petersson, JCP 101, 5900 (1994) Temperature= 298.150000 Pressure= 1.000000 E(ZPE)= 0.033510 E(Thermal)= 0.036677 E(SCF)= -188.836553 DE(MP2)= -0.623990 DE(CBS)= -0.063347 DE(MP34)= -0.011707 DE(CCSD)= -0.014628 DE(Int)= 0.019328 DE(Empirical)= -0.030051 CBS-QB3 (0 K)= -189.527438 CBS-QB3 Energy= -189.524271 CBS-QB3 Enthalpy= -189.523327 CBS-QB3 Free Energy= -189.551515 |
甲酸根气相自由能计算
1 2 3 4 5 6 7 8 9 10 11 12 | %chk=formic_base_CBS.chk #p CBS-QB3 base CBS-QB3 -1 1 C -0.00006100 0.31293500 0.00006900 O -1.13445400 -0.20806200 -0.00001800 O 1.13449900 -0.20804200 -0.00001800 H 0.00000500 1.45122700 -0.00013000 !任何空白行不可省略 |
结果:
1 2 3 4 5 6 7 8 9 10 11 12 13 14 | Complete Basis Set (CBS) Extrapolation: M. R. Nyden and G. A. Petersson, JCP 75, 1843 (1981) G. A. Petersson and M. A. Al-Laham, JCP 94, 6081 (1991) G. A. Petersson, T. Tensfeldt, and J. A. Montgomery, JCP 94, 6091 (1991) J. A. Montgomery, J. W. Ochterski, and G. A. Petersson, JCP 101, 5900 (1994) Temperature= 298.150000 Pressure= 1.000000 E(ZPE)= 0.019583 E(Thermal)= 0.022558 E(SCF)= -188.267139 DE(MP2)= -0.636515 DE(CBS)= -0.066190 DE(MP34)= -0.006145 DE(CCSD)= -0.016029 DE(Int)= 0.020298 DE(Empirical)= -0.029888 CBS-QB3 (0 K)= -188.982025 CBS-QB3 Energy= -188.979049 CBS-QB3 Enthalpy= -188.978105 CBS-QB3 Free Energy= -189.005820 |
甲酸溶剂化自由能计算
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 | %chk=FORMIC_solv.chk #p HF/6-311+g(2d,p) scrf=(smd,solvent=water,read,externaliteration,dovacuum) Structure: formic acid; Opt freq at hf/6-311+g(2d,p) level 0 1 C -0.13383400 0.39594000 0.00002200 O -1.13283000 -0.26239400 -0.00000900 O 1.11497600 -0.08928500 -0.00000700 H -0.10360900 1.49434600 0.00003500 H 1.04945100 -1.05655300 -0.00003800 cav dis rep !任何空白行不可省略 |
结果:
1 2 3 4 5 6 7 8 9 10 11 12 13 14 | Total free energy in solution: - with all non electrostatic terms (a.u.) = -188.845427 -------------------------------------------------------------------- (Unpolarized solute)-Solvent (kcal/mol) = -9.92 (Polarized solute)-Solvent (kcal/mol) = -13.61 Solute polarization (kcal/mol) = 2.01 Total electrostatic (kcal/mol) = -11.60 -------------------------------------------------------------------- Cavitation energy (kcal/mol) = 8.28 Dispersion energy (kcal/mol) = -8.11 Repulsion energy (kcal/mol) = 1.41 Total non electrostatic (kcal/mol) = 1.58 DeltaG (solv) (kcal/mol) = -10.03 -------------------------------------------------------------------- |
甲酸根溶剂化自由能计算
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 | %chk=formic_base_solv-new.chk #p hf/6-311+g(2d,p) scrf=(smd,solvent=water,read,externaliteration,dovacuum) Opt freq at hf/6-311+g(2d,p) level -1 1 C 0.00000477 0.33937336 -0.00001071 O -1.12544578 -0.21806360 0.00000268 O 1.12544806 -0.21805667 0.00000268 H -0.00004688 1.45272204 0.00002144 cav dis rep !任何空白行不可省略 |
结果:
1 2 3 4 5 6 7 8 9 10 11 12 13 14 | Total free energy in solution: - with all non electrostatic terms (a.u.) = -188.383442 -------------------------------------------------------------------- (Unpolarized solute)-Solvent (kcal/mol) = -76.44 (Polarized solute)-Solvent (kcal/mol) = -82.53 Solute polarization (kcal/mol) = 3.28 Total electrostatic (kcal/mol) = -79.25 -------------------------------------------------------------------- Cavitation energy (kcal/mol) = 7.60 Dispersion energy (kcal/mol) = -7.39 Repulsion energy (kcal/mol) = 1.35 Total non electrostatic (kcal/mol) = 1.57 DeltaG (solv) (kcal/mol) = -77.69 -------------------------------------------------------------------- |
五. 参考文献
[1] Matthew D. Liptak and and George C. Shields. Accurate pKa Calculations for Carboxylic Acids Using Complete Basis Set and Gaussian-n Models Combined with CPCM Continuum Solvation Methods. Journal of the American Chemical Society 2001 123 (30), 7314-7319. DOI: 10.1021/ja010534f
六. 相关主题
- Gaussian教程|键解离能的计算
- Gaussian教程|溶剂化自由能的计算
http://blog.molcalx.com.cn/2017/11/05/gaussian-tutorial-bond-dissociation-energy.html
http://blog.molcalx.com.cn/2017/10/10/gaussian-solvation-energy-tutorial.html



